Thomson of Rutherford?
Before Thomson, it was thought that atoms were indivisible chunks of matter with no internal structure. Thomson observed that x-rays could ionize monatomic gasses. Based on this observation, he made the inference that atoms could be separated into a negative part and a positive part. Also, based on the observations that cathode rays could be converted to current and deflected by a magnetic field, he made the inference that cathode rays were made of negative charges, and therefore that the negative charges could be removed from the bulk of the atoms. He then developed a model of the atom in which little electrons were stuck in a big positive goo. Note that he had a lot of evidence for the electrons, but his model of the positive goo was based mainly on a lack of evidence: no one had ever observed positive charges being separated from the atom or observed any evidence of their structure, so he just assumed the positive charge was one big mass.
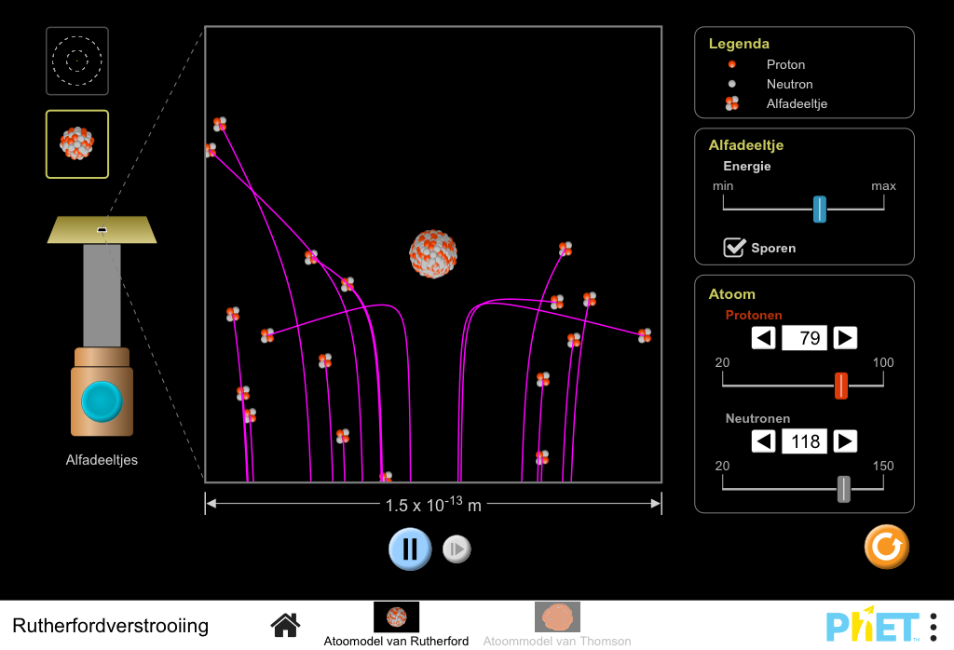
This simulation gives a microscopic picture of Rutherford’s famous experiment in which he shot alpha particles at a thin foil of gold. Based on Thomson’s Plum Pudding model, how did Rutherford expect the alpha particles to behave when he shot them at the gold atoms? (To see this behavior, go to the “Plum Pudding Atom” panel of the simulation.) Why? What did he observe instead? (Go to the “Rutherford Atom” panel.) Based on his observations, what inference did Rutherford make about the distribution of positive charge in the atom?