Radioactive Decay
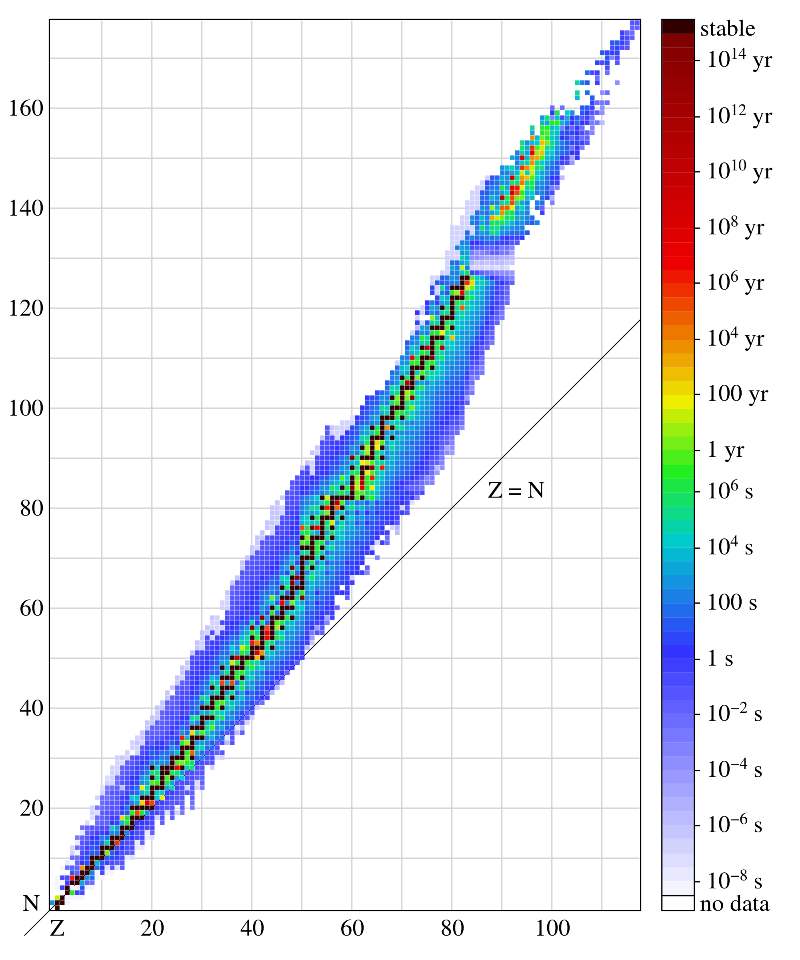
Table of Nuclides
A table of nuclides is a graphical representation of all the stable and unstable isotopes in nature. There is a clear trend that indicates that nuclei of increasing size always have a larger number of neutrons than protons, or N>Z, whereas smaller elements tend to have equal numbers.
Nuclidic Table
Radioactive Decay Constant
When we speak of radioactivity, we just mean that unstable nuclei tend to decay spontaneously in nature. There are many mechanisms of decay. Regardless of the mechanism, there is a corresponding probability of decay for any nuclear species. One way to quantify this is to refer to the decay constant which we will denote . The decay constant is the probability of decay of a nucleus per second. So if , we should expect that each nucleus has a 50/50 chance of decaying within the next second. I wouldn't want to be anywhere near such a source!
The decay constant is constant. This may trouble you. What it means for this to be constant is that if you've been holding a radioactive nucleus with a decay constant that happens to be 0.5 in your hand for a few seconds, it's chance of decay is still 50/50 in the next second. If it doesn't decay still, the probability will also be 50/50 in the subsequent second. While this may seem troubling, the laws of nature obey this principle. What it seems to imply is that there is no "memory" within the nucleus that makes decay more probable later rather than earlier. In analogy, the probability of a prisoner escaping might increase in time because they have time to scheme and to literally chip away at their prison wall and dig a hole. In this sense conditions tomorrow are not the same as today. This has no parallel in nuclei. Rather, it is more like coin tosses. It doesn't matter how many time you flip a coin and get tails back-to-back. The next toss is always 50/50 probability because each toss is an independent, non-causally-linked event. Nuclear decay is like this.
Activity
The activity (as in radioactivity) tells us how actively a sample is decaying. If there are lots of decay events taking place, the activity is high. The activity, where is the number of nuclei in the sample. The activity is in units of decays per second. In mathematical terms, we can then write or the rate of change of our sample size is just the activity. Setting the two views of activity equal allows us to write a differential equation which will allow us to find the sample size that we should expect to be present after a time t: . This equation may be rewritten in a form that will allow solution according to the method of separation of variables:Integrating both sides and evaluating leads to . In this equation, is the number of nuclei present at t=0. Since activity is directly proportional to N, we may also choose to write , where is the activity at t=0. This indicates that only as the sample size goes to zero will the radioactivity go to zero. This is unfortunate for places like Belarus which was the land most affected by the Chernobyl, Ukraine nuclear reactor explosion of 1986, or more recently the 2011 Fukushima, Japan nuclear disaster. In the news today as I write this in the year 2016 (5 years after the earthquake/tsunami triggered the meltdown) they are still frantically searching for 600 tons of highly radioactive material.
The equations of radioactivity are often rewritten in terms of a half-life. The half-life is the time corresponding to a decrease in sample size (or activity) of a factor of 2. The half-life will be a constant for a given radioisotope just as the decay constant is. To express the time-dependent N or A in terms of half-life simply requires us to find the time at which . Solving the expression leads to the half life, . After a period corresponding to a half-life the sample size will be reduced by a factor of 2. After two half lives it will be reduced by 4, then after three half lives, by 8, etc.
Nuclear Stability
At a close range of roughly or closer, protons and neutrons are very strongly attracted to one another. This force suddenly diminishes outside of this range. Protons, however, being positively charged, will repel one another more and more strongly at close range due to electrostatic repulsion. Thus, there are competing forces inside a nucleus full of protons. The fact that like charges repel tries to drive all protons apart, and the fact that they are attracted by a strong nuclear force tries to keep them together.
If there are too many protons, the long-range electrostatic repulsion will win over the strong nuclear force and will make the nucleus unstable. The role of neutrons, since they don't repel each other nor the protons, and yet still attract both, is to act a bit like nuclear glue. They provide physical separation between protons (although it's not really good to think of them like that) as well as providing more "glue" to hold the overall nucleus together. With this description, it might seem like there would never be a problem with having too many neutrons in a nucleus. The only caveat is that neutrons are unstable. We will continue discussions of this instability in the next section.